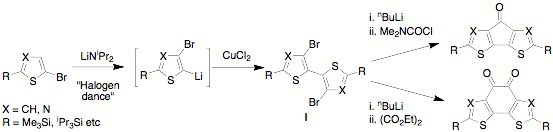
Planar electron-accepting π-systems are useful building blocks for both nonlinear optical and organic electronic applications.
We have recently developed a convenient synthesis of dibromo bithiophenes and bithiazoles of type I (see below) that relies
upon the base-catalyzed “Halogen dance”, in which the lithiation initially takes place adjacent to the bromine atom, before
changing places with it. Compounds I can be converted to a variety of tricyclic species including the keto and diketo species
shown on the right. The structures of representative examples of intermediates and products have been confirmed by X-ray
crystallography.
These species are strong electron acceptors, with dithiazole diketone (lower right) being particularly easy to reduce
(–0.9 V vs. ferrocene) suggesting possible applications in n-channel organic field-effect transistors. We are currently
working on incorporating these cores into more extending π acceptor structures.